

Of note, the Oh-BER-load Deals are available from participating Honda dealerships nationwide. This in turn is open to customers who have missed prior service visits in the past year. Last but not least is a Php 500 savings on periodic maintenance and general repairs for a minimum Php 2,000 amount spent.
The said promo is open to Honda car owners. This is accessed via HCPI’s official website, and all a customer has to do to get a quote is input a car’s 17-digit chassis number. The discount will be based on the quotation generated by the brand’s online Periodic Maintenance Cost Calculator. This is open to Honda owners whose cars have surpassed their warranty periods. The said after-market deal also has a 20 percent discount on periodic maintenance parts and fluids. This is particularly notable for Honda owners who will embark on out-of-town trips to visit loved ones. This likewise includes a 10 percent discount for recommended replacement parts like brake pads/shoes, wipers, and tires.
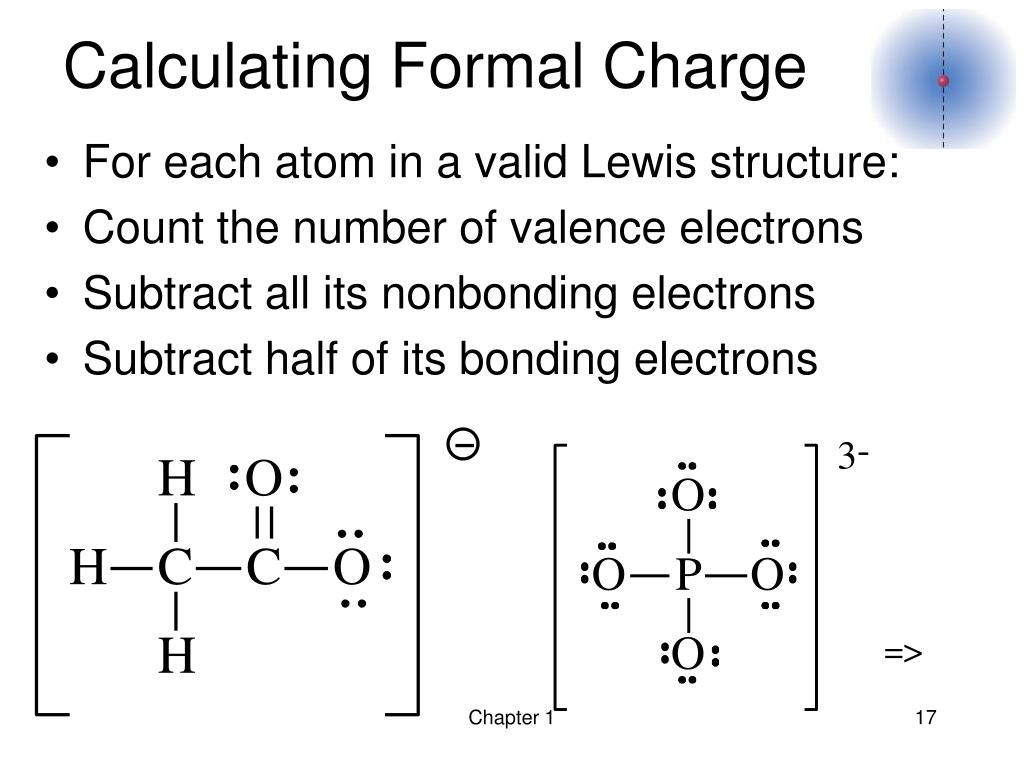
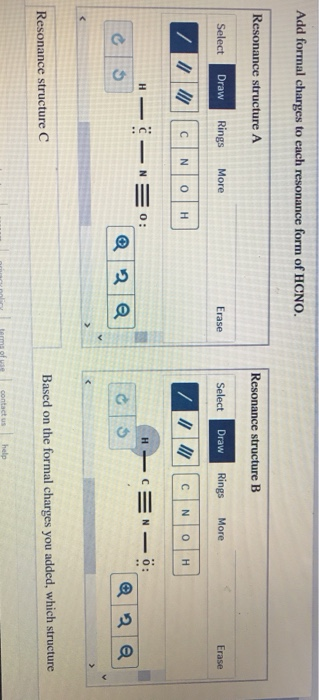
#Calculating formal charge hcno free#
This promo from Honda Cars PH will prepare your Honda just in time for the holiday seasonĬovered by the Oh-BER-load Deals is a free 50-point check-up with advanced tire and battery assessment. It offers plenty of maintenance-related goodies to Honda owners, and it is available from September 11 to December 11, 2023. (HCPI) is introducing the Oh-BER-load Deals after-sales promo. structures that have the same energy as each other.įinally, see how structure II leaves carbon without an octet? You may have learned that atoms prefer to satisfy the octet rule.īecause of a lack of that here, structure II is the most minor resonance contributor (least stable) of these four.To aid their customers in preparing for their upcoming holiday season road trips, Honda Cars Philippines, Inc. Those would be known as degenerate structures, i.e. That automatically means III and IV are the minor resonance contributors (second-most stable).Īlso, notice how structures III and IV are actually identical just reflect them along a vertical axis and they are the same. That means I is the major resonance contributor. We start at I and continuously move clockwise.įirst, see how structures III and IV do not have minimized formal charges? Although there is net charge cancellation, nitrogen, being less electronegative than oxygen, tends less to pull electrons towards it therefore, the major (most stable) resonance structure gives the most electron density to oxygen. The curved arrows indicate the movement of electrons that lead to a new resonance contributor. Let's suppose that we examine the resonance structures of urea, #"H"_2"NCONH"_2#. The most stable structural "snapshots" of a molecule's electron distribution is its major resonance structure. So overall, that's why resonance structures that represent the most stable state of a molecule are the ones that occur most often. To achieve the smallest amount of water in multiple glasses, you should get the same amount in all glasses, not pour it all into one glass. You can also analogize electron delocalization with glasses of water. The more room (orbitals) the electrons have available to move, the more distributed their kinetic energy can be, and in some sense, the less energy "buildup" there would be in select orbitals. Similarly, molecules don't want to be overly excited/hyper, and instead want to achieve the minimum energy, or ground-state energy.ĭelocalizing the electrons in a system with many #pi# electrons helps make that happen in molecules that we draw as resonance structures. So, you drink the minimum amount of coffee so you can just stay awake. If you drink too much coffee in the morning, you might get too hyper over the course of the day, and I don't think anyone really wants to be overly hyper. Minimum energy is analogous to not drinking too much coffee in the morning. Molecules always strive for achieving the minimum energy, whether through electronic relaxations, electron delocalization, or other processes. It's not that certain resonance structures are stable because they occur most often, but that the resonance structures that represent the most stable state of a molecule occur most often. We need to be careful of the cause/effect of this.


 0 kommentar(er)
0 kommentar(er)
